5.4 Entropy Changes in an Ideal Gas
[VW, S & B: 6.5- 6.6, 7.1]
Many aerospace applications involve flow of gases (e.g., air) and we thus examine the entropy relations for ideal gas behavior. The starting point is form (a) of the combined first and second law,
For an ideal gas, ![]() . Thus
. Thus
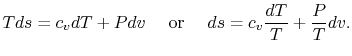
Using the equation of state for an ideal gas (![]() ), we can write the entropy change as an expression with only exact differentials:
), we can write the entropy change as an expression with only exact differentials:
We can think of Equation (5.2) as relating the fractional change in temperature to the fractional change of volume, with scale factors ![]() and
and ![]() ; if the volume increases without a proportionate decrease in temperature (as in the case of an adiabatic free expansion), then
; if the volume increases without a proportionate decrease in temperature (as in the case of an adiabatic free expansion), then ![]() increases. Integrating Equation (5.2) between two states ``1'' and ``2'':
increases. Integrating Equation (5.2) between two states ``1'' and ``2'':
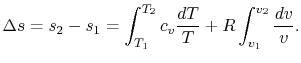
For a perfect gas with constant specific heats
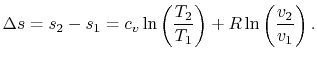
In non-dimensional form (using ![]() )
)
Equation 5.3 is in terms of specific quantities. For ![]() moles of gas,
moles of gas,
![$\displaystyle \frac{\Delta S}{C_v}=N\left[\ln\left(\frac{T_2}{T_1}\right)+(\gamma-1)\ln\left(\frac{V_2}{V_1}\right)\right].$](http://web.mit.edu/16.unified/www/FALL/thermodynamics/notes/img568.png)
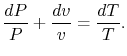
![$\displaystyle ds = c_v\left[\frac{dP}{P}+\frac{dv}{v}\right] + R\frac{dv}{v},$](http://web.mit.edu/16.unified/www/FALL/thermodynamics/notes/img573.png)
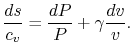
Using both sides of (5.4) as exponents we obtain
Equation (5.5) describes a general process. For the specific situation in which ![]() , i.e., the entropy is constant, we recover the expression
, i.e., the entropy is constant, we recover the expression ![]() . It was stated that this expression applied to a reversible, adiabatic process. We now see, through use of the second law, a deeper meaning to the expression, and to the concept of a reversible adiabatic process, in that both are characteristics of a constant entropy, or isentropic, process.
. It was stated that this expression applied to a reversible, adiabatic process. We now see, through use of the second law, a deeper meaning to the expression, and to the concept of a reversible adiabatic process, in that both are characteristics of a constant entropy, or isentropic, process.
Muddy Points
Why do you rewrite the entropy change in terms of ![]() ? (MP 5.4)
? (MP 5.4)
What is the difference between isentropic and adiabatic? (MP 5.5)
Disusun Ulang Oleh:
Arip Nurahman
Pendidikan Fisika, FPMIPA. Universitas Pendidikan Indonesia
&
Follower Open Course Ware at MIT-Harvard University. Cambridge. USA.
Materi kuliah termodinamika ini disusun dari hasil perkuliahan di departemen fisika FPMIPA Universitas Pendidikan Indonesia dengan Dosen:
1. Bpk. Drs. Saeful Karim, M.Si.
2. Bpk. Insan Arif Hidayat, S.Pd., M.Si.
Dan dengan sumber bahan bacaan lebih lanjut dari :
Massachusetts Institute of Technology, Thermodynamics
Professor Z. S. Spakovszk, Ph.D.
Office: 31-265
Phone: 617-253-2196
Email: zolti@mit.edu
Aero-Astro Web: http://mit.edu/aeroastro/people/spakovszky
Gas Turbine Laboratory: home
Ucapan Terima Kasih:Kepada Para Dosen di MIT dan Dosen Fisika FPMIPA Universitas Pendidikan Indonesia
Semoga Bermanfaat
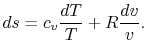
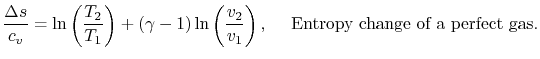
![$\displaystyle \frac{\Delta s}{c_v}=\ln\left(\frac{P_2}{P_1}\right)+\gamma\ln\le... ...v_1}\right) =ln\left[\frac{P_2}{P_1}\left(\frac{v_2}{v_1}\right)^\gamma\right].$](http://web.mit.edu/16.unified/www/FALL/thermodynamics/notes/img575.png)
![$\displaystyle \frac{P_2v_2^\gamma}{P_1 v_1^\gamma}=\left[Pv^\gamma\right]^2_1=e^{\Delta s/c_v}.$](http://web.mit.edu/16.unified/www/FALL/thermodynamics/notes/img576.png)


Tidak ada komentar:
Posting Komentar