6.1 Limitations on the Work that Can be Supplied by a Heat Engine
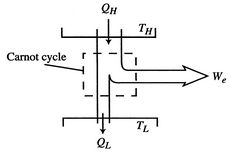
The second law enables us to make powerful and general statements concerning the maximum work that can be derived from any heat engine which operates in a cycle. To illustrate these ideas, we use a Carnot cycle which is shown schematically in Figure 6.1.
The engine operates between two heat reservoirs, exchanging heat ![]() with the high temperature reservoir at
with the high temperature reservoir at ![]() and
and ![]() with the reservoir at
with the reservoir at ![]() . The entropy changes of the two reservoirs are
. The entropy changes of the two reservoirs are
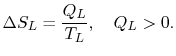 0.$">
0.$">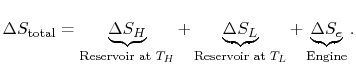
For the engine we can write the first law as
Hence, using (6.1)
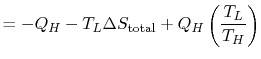 | ||
![$\displaystyle =-Q_H\left[1-\left(\frac{T_L}{T_H}\right)\right]-T_L\Delta S_\textrm{total}.$](http://web.mit.edu/16.unified/www/FALL/thermodynamics/notes/img642.png) |
The work of the engine can be expressed in terms of the heat received by the engine as
![$\displaystyle W_e=Q_{He}\left[1-\left(\frac{T_L}{T_H}\right)\right]-T_L\Delta S_\textrm{total}.$](http://web.mit.edu/16.unified/www/FALL/thermodynamics/notes/img643.png)
![$\displaystyle \textrm{Maximum work for an engine working between $T_H$ and $T_L$:}\quad W_e =Q_{He}\left[1-\left(\frac{T_L}{T_H}\right)\right].$](http://web.mit.edu/16.unified/www/FALL/thermodynamics/notes/img645.png)
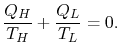
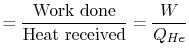 | ||
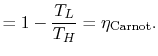 |
The Carnot efficiency is thus the maximum efficiency that can occur in an engine working between two given temperatures.
We can approach this last point in another way. The engine work is given by
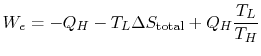
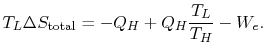
![$\displaystyle \Delta S_\textrm{total} = \frac{Q_{He}}{T_L}\left[1-\frac{T_L}{T_... ...c{Q_{He}}{T_L}\biggl[\eta_\textrm{Carnot}-\eta_\textrm{Any other cycle}\biggr].$](http://web.mit.edu/16.unified/www/FALL/thermodynamics/notes/img652.png)
Muddy Points
So, do we lose the capability to do work when we have an irreversible process and entropy increases? (MP 6.1)
Why do we study cycles starting with the Carnot cycle? Is it because it is easier to work with? (MP 6.2)
Disusun Ulang Oleh:
Arip Nurahman
Pendidikan Fisika, FPMIPA. Universitas Pendidikan Indonesia
&
Follower Open Course Ware at MIT-Harvard University. Cambridge. USA.
Materi kuliah termodinamika ini disusun dari hasil perkuliahan di departemen fisika FPMIPA Universitas Pendidikan Indonesia dengan Dosen:
1. Bpk. Drs. Saeful Karim, M.Si.
2. Bpk. Insan Arif Hidayat, S.Pd., M.Si.
Dan dengan sumber bahan bacaan lebih lanjut dari :
Massachusetts Institute of Technology, Thermodynamics
Professor Z. S. Spakovszk, Ph.D.
Office: 31-265
Phone: 617-253-2196
Email: zolti@mit.edu
Aero-Astro Web: http://mit.edu/aeroastro/people/spakovszky
Gas Turbine Laboratory: home
Ucapan Terima Kasih:Kepada Para Dosen di MIT dan Dosen Fisika FPMIPA Universitas Pendidikan Indonesia
Semoga Bermanfaat
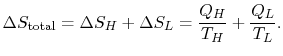


Tidak ada komentar:
Posting Komentar