Disusun Ulang Oleh:
Arip Nurahman
Pendidikan Fisika, FPMIPA. Universitas Pendidikan Indonesia
&
Follower Open Course Ware at MIT-Harvard University. Cambridge. USA.
Materi kuliah termodinamika ini disusun dari hasil perkuliahan di departemen fisika FPMIPA Universitas Pendidikan Indonesia dengan Dosen:
1. Bpk. Drs. Saeful Karim, M.Si.
2. Bpk. Insan Arif Hidayat, S.Pd., M.Si.
Dan dengan sumber bahan bacaan lebih lanjut dari :
Massachusetts Institute of Technology, Thermodynamics
Professor Z. S. Spakovszk, Ph.D.
Office: 31-265
Phone: 617-253-2196
Email: zolti@mit.edu
Aero-Astro Web: http://mit.edu/aeroastro/people/spakovszky
Gas Turbine Laboratory: home
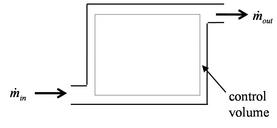
2.5 Control volume form of the conservation laws
[VWB&S, 6.1, 6.2]The thermodynamic laws (as well as Newton's laws) are for a system, a specific quantity of matter. More often, in propulsion and power problems, we are interested in what happens in a fixed volume, for example a rocket motor or a jet engine through which mass is flowing at a certain rate. We may also be interested in the rates of heat and work into and out of a system. For this reason, the control volume form of the system laws is of great importance. A schematic of the difference is shown in Figure 2.8. Rather than focus on a particle of mass which moves through the engine, it is more convenient to focus on the volume occupied by the engine. This requires us to use the control volume form of the thermodynamic laws, developed below.
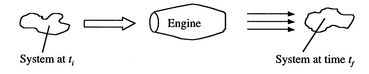 |
2.5.1 Conservation of mass
For the control volume shown, the rate of change of mass inside the volume is given by the difference between the mass flow rate in and the mass flow rate out. For a single flow coming in and a single flow coming out this is
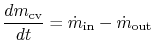 | |
If the mass inside the control volume changes with time it is because some mass is added or some is taken out. In the special case of a steady flow,
| |
 |
2.5.2 Conservation of energy
The first law of thermodynamics can be written as a rate equation:
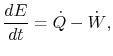
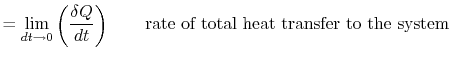 | | |
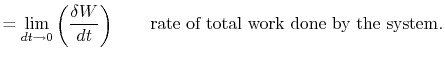 | |
To derive the first law as a rate equation for a control volume we proceed as with the mass conservation equation. The physical idea is that any rate of change of energy in the control volume must be caused by the rates of energy flow into or out of the volume. The heat transfer and the work are already included and the only other contribution must be associated with the mass flow in and out, which carries energy with it. Figure 2.10 shows two schematics of this idea. The desired form of the equation will be
| |
| [Simple] 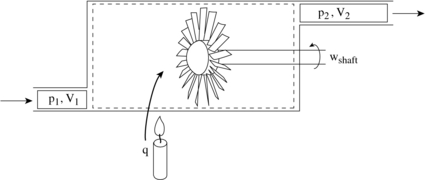 [More General] [More General] 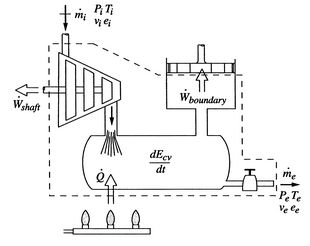 |
The fluid that enters or leaves has an amount of energy per unit mass given by
Including all possible energy flows (heat, shaft work, shear work, piston work, etc.), the first law can then be written as:
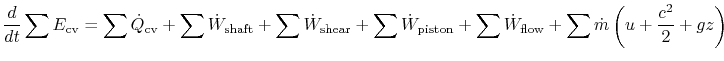
We can then combine the specific internal energy term, ![]() , in
, in ![]() and the specific flow work term,
and the specific flow work term, ![]() , to make the enthalpy appear:
, to make the enthalpy appear:
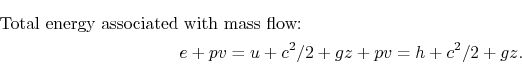
Thus, the first law can be written as:
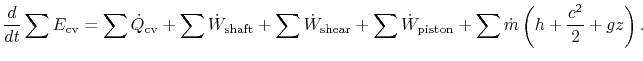
For most of the applications in this course, there will be no shear work and no piston work. Hence, the first law for a control volume will be most often used as:
Note how our use of enthalpy has simplified the rate of work term. In writing the control volume form of the equation we have assumed only one entering and one leaving stream, but this could be generalized to any number of inlet and exit streams.
In the special case of a steady-state flow,
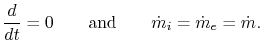 | |
Applying this to Equation 2.10 produces a form of the ``Steady Flow Energy Equation'' (SFEE),
which has units of Joules per second. We could also divide by the mass flow to produce
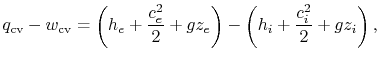 | |
which has units of Joules per second per kilogram. For problems of interest in aerospace applications the velocities are high and the term that is associated with changes in the elevation is small. From now on, we will neglect the
Muddy Points
What is shaft work? (MP 2.5)
What distinguishes shaft work from other works? (MP 2.6)
Definition of a control volume (MP 2.7)
2.5.3 Stagnation Temperature and Stagnation Enthalpy
Suppose that our steady flow control volume is a set of streamlines describing the flow up to the nose of a blunt object, as in Figure 2.11.
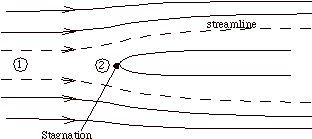 |
The streamlines are stationary in space, so there is no external work done on the fluid as it flows. If there is also no heat transferred to the flow (adiabatic), then the steady flow energy equation becomes
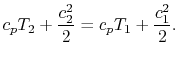 | |
The quantity that is conserved is defined as the stagnation temperature,
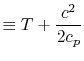 | | |
or | ||
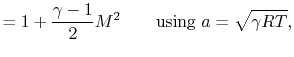 | | |
where
It is also convenient to define the stagnation enthalpy,
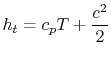 | |
which allows us to write the Steady Flow Energy Equation in a simpler form as
| |
Note that for a quasi-static adiabatic process
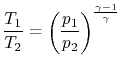 | |
so we can write
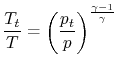 | |
and define the relationship between stagnation pressure and static pressure as
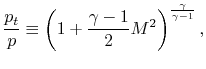 | |
where, the stagnation pressure is the pressure that the fluid would reach if it were brought to zero speed, via a steady, adiabatic, quasi-static process with no external work.
2.5.3.1 Frame dependence of stagnation quantities
An area of common confusion is the frame dependence of stagnation quantities. The stagnation temperature and stagnation pressure are the conditions the fluid would reach if it were brought to zero speed relative to some reference frame, via a steady adiabatic process with no external work (for stagnation temperature) or a steady, adiabatic, reversible process with no external work (for stagnation pressure). Depending on the speed of the reference frame the stagnation quantities will take on different values.
For example, consider a high speed reentry vehicle traveling through the still atmosphere, which is at temperature, ![]() . Let's place our reference frame on the vehicle and stagnate a fluid particle on the nose of the vehicle (carrying it along with the vehicle and thus essentially giving it kinetic energy). The stagnation temperature of the air in the vehicle frame is
. Let's place our reference frame on the vehicle and stagnate a fluid particle on the nose of the vehicle (carrying it along with the vehicle and thus essentially giving it kinetic energy). The stagnation temperature of the air in the vehicle frame is
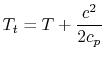 | |
where
The confusion comes about because ![]() is usually referred to as the static temperature. In common language this has a similar meaning as ``stagnation,'' but in fluid mechanics and thermodynamics static is used to label the thermodynamic properties of the gas (
is usually referred to as the static temperature. In common language this has a similar meaning as ``stagnation,'' but in fluid mechanics and thermodynamics static is used to label the thermodynamic properties of the gas (![]() ,
, ![]() , etc.), and these are not frame dependent.
, etc.), and these are not frame dependent.
Thus in our re-entry vehicle example, looking at the still atmosphere from the vehicle frame we see a stagnation temperature hotter than the atmospheric (static) temperature. If we look at the same still atmosphere from a stationary frame, the stagnation temperature is the same as the static temperature.
2.5.3.2 Example
For the case shown below, a jet engine is sitting motionless on the ground prior to take-off. Air is entrained into the engine by the compressor. The inlet can be assumed to be frictionless and adiabatic.
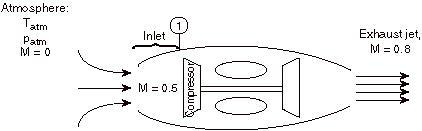 |
Considering the state of the gas within the inlet, prior to passage into the compressor, as state (1), and working in the reference frame of the motionless airplane:
- Is
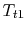 greater than, less than, or equal to
greater than, less than, or equal to 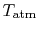 ?
? The stagnation temperature of the atmosphere,
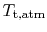 , is equal to
, is equal to 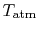 since it is moving the same speed as the reference frame (the motionless airplane). The steady flow energy equation tells us that if there is no heat or shaft work (the case for our adiabatic inlet) the stagnation enthalpy (and thus stagnation temperature for constant
since it is moving the same speed as the reference frame (the motionless airplane). The steady flow energy equation tells us that if there is no heat or shaft work (the case for our adiabatic inlet) the stagnation enthalpy (and thus stagnation temperature for constant 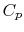 ) remains unchanged. Thus
) remains unchanged. Thus 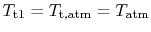
- Is
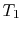 greater than, less than, or equal to
greater than, less than, or equal to 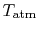 ?
? If
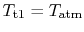 then
then 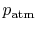 ?
? Equal, by the same argument as 1.
- Is
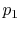 greater than, less than, or equal to
greater than, less than, or equal to 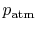 ?
? Less than, by the same argument as 2.
2.5.3.3 Steady Flow Energy Equation in terms of Stagnation Enthalpy
The form of the ``Steady Flow Energy Equation'' (SFEE) that we will most commonly use is Equation 2.11 written in terms of stagnation quantities, and neglecting chemical and potential energies,
Muddy Points
What is the difference between enthalpy and stagnation enthalpy? (MP 2.8)
2.5.4 Example Applications of the First Law of Thermodynamics
2.5.4.1 Tank Filling
Using what we have just learned we can attack the tank filling problem solved in Section 2.3.3 from an alternate point of view using the control volume form of the first law. In this problem the shaft work is zero, and the heat transfer, kinetic energy changes, and potential energy changes are neglected. In addition there is no exit mass flow.
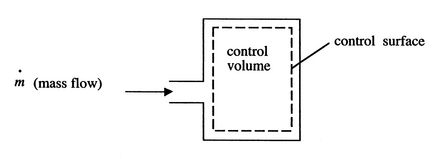 |
The control volume form of the first law is therefore
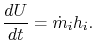
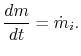
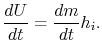
2.5.4.2 Flow through a rocket nozzle
A liquid bi-propellant rocket consists of a thrust chamber and nozzle and some means for forcing the liquid propellants into the chamber where they react, converting chemical energy to thermal energy.
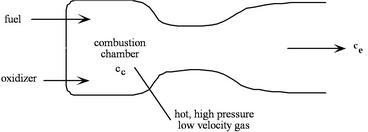 |
Once the rocket is operating we can assume that all of the flow processes are steady, so it is appropriate to use the steady flow energy equation. Also, for now we will assume that the gas behaves as a perfect gas with constant specific heats, though in general this is a poor approximation. There is no external work, and we assume that the flow is adiabatic. We define our control volume as going between location ![]() , in the chamber, and location
, in the chamber, and location ![]() , at the exit, and then write the First Law as
, at the exit, and then write the First Law as
| |
or
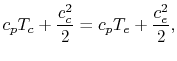 | |
Therefore
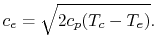 | |
If we assume quasi-static, adiabatic expansion then
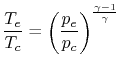 | |
so
![$\displaystyle c_e = \sqrt{2 c_p T_c \left[1 - \left(\frac{p_e}{p_c}\right)^{\frac{\gamma-1}{\gamma}}\right]}.$](http://web.mit.edu/16.unified/www/FALL/thermodynamics/notes/img300.png) | |
2.5.4.3 Power to drive a gas turbine compressor
Consider for example the PW4084 pictured in Figure 2.15. The engine is designed to produce about 84,000 lbs of thrust at takeoff. The engine is a two-spool design. The fan and low pressure compressor are driven by the low pressure turbine. The high pressure compressor is driven by the high pressure turbine. We wish to find the total shaft work required to drive the compression system.
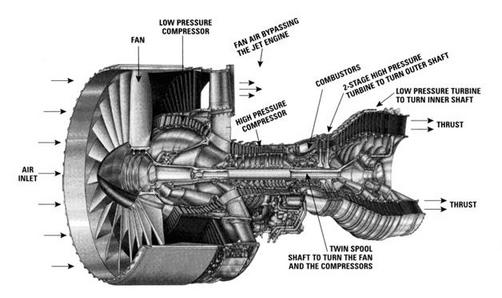 |
| | |||
| | |||
| | |||
| | |||
| |
We define our control volume to encompass the compression system, from the front of the fan to the back of the fan and high pressure compressor, with the shaft cutting through the back side of the control volume. Heat transfer from the gas streams is negligible, so we write the First Law (steady flow energy equation) as:
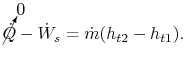 | |
For this problem we must consider two streams, the fan stream,
| | ||
| |
We obtain the temperature change by assuming that the compression process is quasi-static and adiabatic,
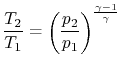 | |
then
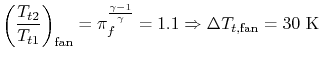 | |
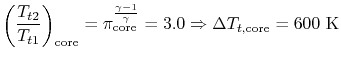 | |
Substituting these values into the expression for the first law above, along with estimates of
| | ||
| | ||
| |
Note that
Ucapan Terima Kasih:
Kepada Para Dosen di MIT dan Dosen Fisika FPMIPA Universitas Pendidikan Indonesia
Semoga Bermanfaat
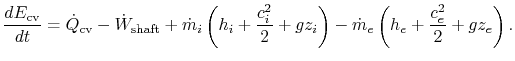
![$\displaystyle \dot{Q}_{\textrm{cv}} - \dot{W}_{\textrm{cv}} = \dot{m}\left[\left(h_e+\frac{c_e^2}{2}+gz_e\right)-\left(h_i+\frac{c_i^2}{2}+gz_i\right)\right],$](http://web.mit.edu/16.unified/www/FALL/thermodynamics/notes/img263.png)


Tidak ada komentar:
Posting Komentar