7.4 Connection between the Statistical Definition of Entropy and Randomness
We need now to examine the behavior of the statistical definition of entropy as regards randomness. Because a uniform probability distribution reflects the largest randomness, a system with ![]() allowed states will have the greatest entropy when each state is equally likely. In this situation, the probabilities become
allowed states will have the greatest entropy when each state is equally likely. In this situation, the probabilities become
where
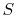 is maximum when
is maximum when 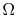 is maximum, which means many permitted quantum states, hence much randomness,
is maximum, which means many permitted quantum states, hence much randomness,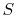 is minimum when
is minimum when 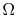 is minimum. In particular, for
is minimum. In particular, for 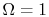 , there is no randomness and
, there is no randomness and 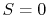 .
.
We can also examine the additive property of entropy with respect to probabilities. If we have two systems, ![]() and
and ![]() , which are viewed as a combined system,
, which are viewed as a combined system, ![]() , the quantum states for the combined system are the combinations of the quantum states from
, the quantum states for the combined system are the combinations of the quantum states from ![]() and
and ![]() . The quantum state where
. The quantum state where ![]() is in its state
is in its state ![]() and
and ![]() is in its state
is in its state ![]() would have a probability
would have a probability ![]() because the two probabilities are independent. The number of probabilities for the combined system,
because the two probabilities are independent. The number of probabilities for the combined system, ![]() , is thus defined by
, is thus defined by ![]() . The entropy of the combined system is
. The entropy of the combined system is
Equation (7.16) is sometimes taken as the basic definition of entropy, but it should be remembered that it is only appropriate when each quantum state is equally likely. Equation (7.12) is more general and applies equally for equilibrium and non-equilibrium situations.
A simple numerical example shows trends in entropy changes and randomness for a system which can exist in three states. Consider the five probability distributions
| | ||||||||
| | ||||||||
| | ||||||||
| | ||||||||
![$\displaystyle S=-3k\left[\frac{1}{3}\ln\left(\frac{1}{3}\right)\right]$](http://web.mit.edu/16.unified/www/FALL/thermodynamics/notes/img968.png) | |
Disusun Ulang Oleh:
Arip Nurahman
Pendidikan Fisika, FPMIPA. Universitas Pendidikan Indonesia
&
Follower Open Course Ware at MIT-Harvard University. Cambridge. USA.
Materi kuliah termodinamika ini disusun dari hasil perkuliahan di departemen fisika FPMIPA Universitas Pendidikan Indonesia dengan Dosen:
1. Bpk. Drs. Saeful Karim, M.Si.
2. Bpk. Insan Arif Hidayat, S.Pd., M.Si.
Dan dengan sumber bahan bacaan lebih lanjut dari :
Massachusetts Institute of Technology, Thermodynamics
Professor Z. S. Spakovszk, Ph.D.
Office: 31-265
Phone: 617-253-2196
Email: zolti@mit.edu
Aero-Astro Web: http://mit.edu/aeroastro/people/spakovszky
Gas Turbine Laboratory: home
Ucapan Terima Kasih:Kepada Para Dosen di MIT dan Dosen Fisika FPMIPA Universitas Pendidikan Indonesia
Semoga Bermanfaat
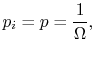
![$\displaystyle = -k \sum_{i=1}^\Omega \frac{1}{\Omega}\ln\left(\frac{1}{\Omega}\... ...ega}\ln\left(\frac{1}{\Omega}\right)\right] =-k\ln\left(\frac{1}{\Omega}\right)$](http://web.mit.edu/16.unified/www/FALL/thermodynamics/notes/img938.png)


Tidak ada komentar:
Posting Komentar