TERMODINAMIKA
Disusun Ulang Oleh:
Arip Nurahman
Pendidikan Fisika, FPMIPA. Universitas Pendidikan Indonesia
&
Follower Open Course Ware at MIT-Harvard University. Cambridge. USA.
Materi kuliah termodinamika ini disusun dari hasil perkuliahan di departemen fisika FPMIPA Universitas Pendidikan Indonesia dengan Dosen:
1. Bpk. Drs. Saeful Karim, M.Si.
2. Bpk. Insan Arif Hidayat, S.Pd., M.Si.
Dan dengan sumber bahan bacaan lebih lanjut dari :
Massachusetts Institute of Technology, Thermodynamics
Professor Z. S. Spakovszk, Ph.D.
Office: 31-265
Phone: 617-253-2196
Email: zolti@mit.edu
Aero-Astro Web: http://mit.edu/aeroastro/people/spakovszky
Gas Turbine Laboratory: home
2.2 Corollaries of the First Law
- Work done in any adiabatic (
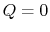 ) process is a function of state. We can write the first law, setting the heat transfer term equal to zero, as
) process is a function of state. We can write the first law, setting the heat transfer term equal to zero, as 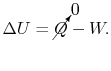
(2..3)
Since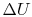 depends only on the state change, now
depends only on the state change, now 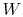 can be found as a function of the state change.
can be found as a function of the state change. - For a cyclic process heat and work transfers are numerically equal
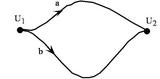
Figure 2.4: Since energy is a function of state only, any process that returns a system to its original state leaves its energy unchanged. 
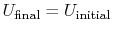
therefore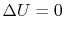
and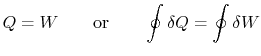
Ucapan Terima Kasih:
Kepada Para Dosen di MIT dan Dosen Fisika FPMIPA Universitas Pendidikan Indonesia
Semoga Bermanfaat


Tidak ada komentar:
Posting Komentar